Catalytic utility of PNN-based MnI pincer complexes in the synthesis of quinolines and transfer hydrogenation of carbonyl derivatives†
Abstract
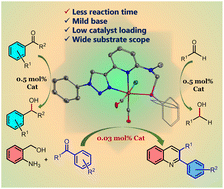
This manuscript describes the synthesis of a triazolyl-pyridine-based phosphine, N-((diphenylphosphaneyl)methyl)-N-methyl-6-(1-phenyl-1H-1,2,3-triazol-4-yl)pyridin-2-amine, [2,6-{(PPh2)CH2N(Me)(C5H3N)(C2HN3C6H5)}] (1) (here onwards referred to as PNN) and its cationic and neutral MnI complexes and catalytic applications. The reaction of 1 with Mn(CO)5Br afforded a cationic complex [Mn(CO)3(PNN)]Br (2), which is highly stable in solid state, but in solution it gradually loses one of the CO groups to form a neutral complex [Mn(CO)2(PNN)Br] (3). Complex 2 on treatment with AgBF4 also yielded a cationic complex [Mn(CO)3(PNN)]BF4 (4). These complexes efficiently promoted the synthesis of quinoline derivatives via acceptor-less dehydrogenative coupling of 2-aminobenzyl alcohol and ketones, with complex 3 showing the highest activity with a very low catalyst loading (0.03 mol%) at 110 °C. Complex 3 (0.5 mol%) also showed excellent catalytic activity in the transfer hydrogenation of ketones and aldehydes to form respective secondary and primary alcohols.
- This article is part of the themed collection: Celebrating International Women’s Day 2025: Women in Inorganic Chemistry


 Please wait while we load your content...
Please wait while we load your content...