On the mechanism of action of arsenoplatins: arsenoplatin-1 binding to a B-DNA dodecamer†
Abstract
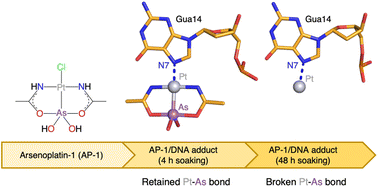
The reaction of Pt-based anticancer agents with arsenic trioxide affords robust complexes known as arsenoplatins. The prototype of this family of anticancer compounds is arsenoplatin-1 (AP-1) that contains an As(OH)2 fragment linked to a Pt(II) moiety derived from cisplatin. Crystallographic and spectrometric studies of AP-1 binding to a B-DNA double helix dodecamer are presented here, in comparison with cisplatin and transplatin. Results reveal that AP-1, cisplatin and transplatin react differently with the DNA model system. Notably, in the AP-1/DNA systems, the Pt–As bond can break down with time and As-containing fragments can be released. These results have implications for the understanding of the mechanism of action of arsenoplatins.
- This article is part of the themed collections: Celebrating International Women’s Day 2025: Women in Inorganic Chemistry and Articles behind our 2024 journal covers


 Please wait while we load your content...
Please wait while we load your content...