Oxidation-derived anticancer potential of sumanene–ferrocene conjugates†
Abstract
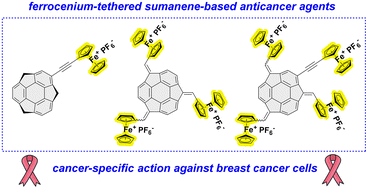
An effective synthetic protocol towards the oxidation of sumanene–ferrocene conjugates bearing one to four ferrocene moieties has been established. The oxidation protocol was based on the transformation of FeII from ferrocene to FeIII-containing ferrocenium cations by means of the treatment of the title organometallic buckybowls with a mild oxidant. Successful isolation of these ferrocenium-tethered sumanene derivatives 5–7 gave rise to the biological evaluation of the first, buckybowl-based anticancer agents, as elucidated by in vitro assays with human breast adenocarcinoma cells (MDA-MB-231) and embryotoxicity trials in zebrafish embryos supported with in silico toxicology studies. The designed ferrocenium-tethered sumanene derivatives featured attractive properties in terms of their use in cancer treatments in humans. The tetra-ferrocenium sumanene derivative 7 featured especially beneficial biological features, elucidated by low (<40% for 10 μM) viabilities of MDA-MB-231 cancer cells together with a 1.4–1.7-fold higher viability of normal cells (human mammary fibroblasts, HMF) for respective concentrations. Compound 7 featured significant cytotoxicity against cancer cells thanks to the presence of sumanene and ferrocenium moieties; the latter motif also provided the selectivity of anticancer action. The biological properties of 7 were also improved in comparison with those of native building blocks, which suggested the effects of the presence of the sumanene skeleton towards the anticancer action of this molecule. Ferrocenium-tethered sumanene derivatives exhibited potential towards the generation of reactive oxygen species (ROS), responsible for biological damage to the cancer cells, with the most efficient generation of the tetra-ferrocenium sumanene derivative 7. Derivative 7 also did not show any embryotoxicity in zebrafish embryos at the tested concentrations, which supports its potential as an effective and cancer-specific anticancer agent. In silico computational analysis also showed no chromosomal aberrations and no mutation with AMES tests for the compound 7 tested with and without microsomal rat liver fractions, which supports its further use as a potent drug candidate in detailed anticancer studies.
- This article is part of the themed collections: Spotlight Collection: Bioinorganic Chemistry and Articles behind our 2024 journal covers


 Please wait while we load your content...
Please wait while we load your content...