Coordination chemistry and FLP reactivity of 1,1- and 1,2-bis-boranes†
Abstract
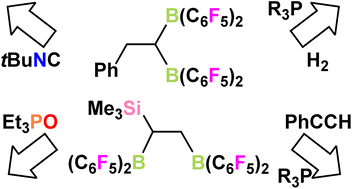
Coordination chemistry and frustrated Lewis pair (FLP) chemistry have been most commonly studied using monodentate Lewis acids. In this paper, we examine the corresponding reactions employing the 1,1- and 1,2-bis-boranes, PhCH2CH(B(C6F5)2)21 and Me3SiCH(B(C6F5)2)CH2B(C6F5)22, respectively. Coordination of isocyanide to these species results in the formation of the products RCH(B(C6F5)2CNtBu)CH2(B(C6F5)2CNtBu) (R = Ph 3, Me3Si 4). The rearrangement of 1 to give the 1,2-bis-borane adduct 3 was probed and attributed to a donor-induced retrohydroboration and subsequent hydroboration. The analogous reaction of 1 is evident in efforts to use the Gutman–Beckett method to assess its Lewis acidity. However, in combination with tBu3P, bis-boranes 1 and 2 form FLPs and react with H2 to give [tBu3PH][PhCH2CH(B(C6F5)2)2(μ-H)] 5a and [tBu3PH][Me3SiCH(B(C6F5)2)CH2(B(C6F5)2)(μ-H)] 6, respectively. Reactions of 1 and 2 with various donors and PhCCH were shown to give deprotonation and addition products, depending on the nature of the base. However, in the case of 1, products resulting from retrohydroboration, and subsequent hydroboration are evident. Several of these alkyne products are crystallographically characterized.


 Please wait while we load your content...
Please wait while we load your content...