Hyphenation of lipophilic ruthenium(ii)-diphosphine core with 5-fluorouracil: an effective metallodrug against glioblastoma brain cancer cells†
Abstract
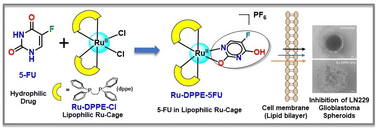
Glioblastoma multiforme (GBM) is the most common highly aggressive malignant brain tumor, with a very limited chance for survival post-diagnosis and post-treatment. Despite significant advancement in GBM genomics implicated in molecularly targeted chemotherapies, the prognosis remains poor and requires new drug discovery approaches. We used fluoropyrimidine 5-fluorouracil (5-FU), an antimetabolite anticancer drug conjugated or ‘caged’ within a lipophilic Ru(II)-diphosphine (dppe) core formulated as [RuII(dppe)2(5-FU)]PF6 (Ru-DPPE-5FU), where dppe = 1,2-bis(diphenylphosphino)ethane, and evaluated its in vitro cytotoxicity in depth with aggressive GBM cells (LN229). The hydrophilic nature of 5-FU limits its passage through the blood–brain barrier (BBB), which prevents its effective accumulation and efficacy for GBM tumors. Herein, we attempted to modulate the lipophilicity of 5-FU by inserting it within a well-designed lipophilic {Ru(dppe)2}-core with anticipated higher efficiency towards GBM. The physicochemical properties of [RuII(dppe)2(5-FU)]PF6 (Ru-DPPE-5FU) were studied using various spectroscopic and analytical techniques. The molecular structure was determined using X-ray crystallography, showing a distorted {RuP4NO} octahedral geometry with bidentate (N, O) binding of 5-FU and its aromatization in the Ru(II)-bound form. The 31P-NMR spectra of Ru-DPPE-5FU showed four closely spaced distinct 31P-signals, indicating four unique chemical environments around P, and the strong coupling constants between them make it a second-order spectrum. The RuII/RuIII redox potential in Ru-DPPE-5FU shifted by ∼0.91 V towards the anodic region as compared to its precursor complex cis-[Ru(dppe)2Cl2] (Ru-DPPE-Cl). DFT-based theoretical calculations have been performed to correlate the experimental electronic absorption spectra and redox behaviours of the complexes. The electrostatic potential (ESP) plots indicate the delocalization of the charge density on the O-/F-atom from the 5-FU ligand towards Ru(II) upon its complexation. The antioxidant properties of all the compounds were quantified by a 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay. The hyphenation of the 5-fluorouracil (5-FU) ligand to the lipophilic {Ru(dppe)2}-core endowed lipophilicity to Ru-DPPE-5FU with higher in vitro cytotoxicity (IC50 = 2.37 μM) against the LN229 GBM cells as compared to the hydrophilic 5-FU, suggesting efficient cellular uptake. Further biological assays indicated that the complex is highly potent in inhibiting significant proliferation and spheroid formation and restricting the migratory potentials of the GBM cells. Increased caspase 3/7 activity and the presence of apoptotic bodies at the center of 3-D GBM spheroids as revealed by AO/EB dual staining indicated a deeper penetration of the lipophilic complex. The Ru-DPPE-5FU complex displayed lower cytotoxicity in HaCaT normal cells (IC50 = 7.27 μM) in comparison to LN229 cancer cells with a selectivity index (S.I.) of ≥3. Overall, the synergism and caging of 5-FU within the hydrophobic {Ru(dppe)2}-core improves the pharmacokinetic profile of Ru-DPPE-5FU as a potent anticancer agent for glioblastoma.
- This article is part of the themed collection: Spotlight Collection: Bioinorganic Chemistry


 Please wait while we load your content...
Please wait while we load your content...