Photochemical dehydrogenative transformation to heterocycles facilitated by an azo/hydrazo redox couple†
Abstract
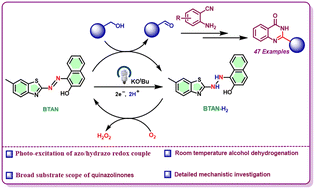
Despite the rich redox chemistry available for the azo motif, studies on its photochemical activation and subsequent application in catalysis are scarce. We design a small organic molecule, benzothiazole azo β-naphthol, which can be reduced under very mild reaction conditions upon photoexcitation. The monoreduced azo promotes hydrogen atom transfer activity to afford dehydrogenation reactions under very mild reaction conditions. Utilizing the molecule as a photocatalyst, we assemble a series of 2-substituted quinazolinone molecules under an aerobic atmosphere. A plethora of alcohols including the challenging aliphatic ones have been successfully dehydrogenated to incorporate as an alkyl group in the quinazolinone. This method is a significant improvement over prior transition metal-catalyzed protocols that needed very high reaction temperatures and sometimes inert atmospheres. A thorough mechanistic investigation involving intermediate isolation, KIE determination, and Stern–Volmer studies delineates the reaction pathway and proves the great efficiency of the monoreduced azo in dehydrogenation reactions.


 Please wait while we load your content...
Please wait while we load your content...