Molecular palladium catalyst enabling efficient electrochemical C–C bond cleavage within lignin model compounds†
Abstract
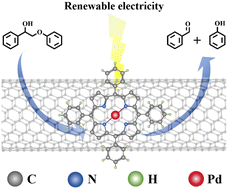
The electrochemical conversion of lignin for the production of high-value aromatic compounds holds immense potential. However, the process of depolymerizing lignin faces significant challenges due to its intricate structure, particularly the existence of C–C bonds, resulting in low conversion and product yields. In this work, we report the successful synthesis of a molecular palladium catalyst and its inaugural application in the electrochemical conversion of lignin model compounds. This catalyst with well-defined coordination structure demonstrates exceptional efficacy and achieves a remarkable 99% conversion rate for the lignin model compound 2-phenoxy-1-phenylethanol with a yield of 32% of the benzaldehyde product while valuable hydrogen is produced at the electrode under ambient conditions. Furthermore, employing radical trapping and EPR test point that the tBuO˙ radical was the key to inducing C–C cleavage for high conversion and enabled a comprehensive elucidation of the possible reaction pathways involving radical/radical cross-coupling. Based on this study, we believe that molecular catalysts will open a new chapter in the electrocatalytic conversion of lignin.


 Please wait while we load your content...
Please wait while we load your content...