A general and expedient amination of alcohols catalysed by a single-site (NN)Co(ii)-bidentate complex under solventless conditions†
Abstract
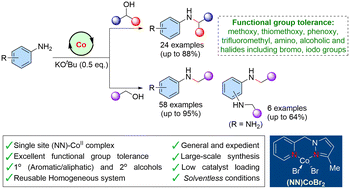
Here we designed and synthesized a NN–CoII bidentate complex and efficiently used it for general and expedient amination of alcohols under benign, solventless conditions. Both primary (including unactivated aliphatic) alcohols and sterically hindered secondary alcohols exhibited very good reactivity and provided diverse amines with good substrate scope (88 examples; up to 95% yields) and excellent functional group tolerance (methoxy, thiomethoxy, phenoxy, trifluoromethyl, amino, alcoholic and halides including bromo and iodo groups). Furthermore, a sequential bis-N-alkylation of diamines was also demonstrated. It was observed that the pyrazole moiety in the ligand backbone plays a crucial role in the amination reaction. Very interestingly, the reusability of the present homogeneous cobalt catalyst was successfully demonstrated.


 Please wait while we load your content...
Please wait while we load your content...