Theoretical study on the degradation mechanism of perfluoro-ethanesulfonic acid under subcritical hydrothermal alkaline conditions
Abstract
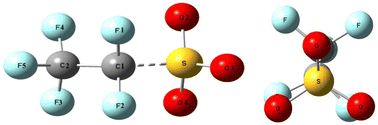
Perfluorosulfonic acid, a widely recognized persistent organic pollutant, has attracted significant attention due to its severe environmental contamination, necessitating urgent resolution. To discover effective degradation strategies, this study implemented density functional theory, utilizing Gaussian 09 software with the WB97XD/6-311++G(2d,2p)//CCSD(T)/6-311++G(2df,2p) computational approach to conduct an in-depth reaction pathway analysis of perfluoroethane sulfonic acid (PFEtS) under subcritical hydrothermal alkaline conditions. It was revealed that PFEtS exhibits an uneven electron density distribution along the carbon chain backbone, with the bond energy of the C2–F4 bond being the lowest, followed by the C1–F1 bond, and the S–C1 bond energy being lower than those of C1–C2 and C–F bonds, rendering it susceptible to breakage. Based on these observations, seven potential degradation pathways of PFEtS were proposed under subcritical hydrothermal alkaline conditions, following optimization, and five reaction pathways have been identified. The degradation process unfolds in two stages. Initially, hydroxyl groups replace the sulfonate in PFEtS to form perfluoroethanol. Subsequently, full mineralization is achieved under alkaline conditions. The most probable reaction pathway involves hydroxyl groups attacking the C1 position, resulting in the generation of CO2 and inorganic fluoride ions. The first step of the reaction is the rate-determining step, with a theoretical rate constant calculated to be 8.41 × 10−5 L mol−1 s−1. This theoretical value is in close agreement with the experimentally determined degradation rate constant of perfluorooctane sulfonate under identical conditions, which is 8.67 × 10−4 L mol−1 s−1. This finding corroborates the experimental observation that longer-chain perfluoro-sulfonates degrade faster than their shorter-chain counterparts.


 Please wait while we load your content...
Please wait while we load your content...