Theoretical structural and spectroscopic characterization of peroxyacetic acid (CH3–CO–OOH): study of the far infrared region†
Abstract
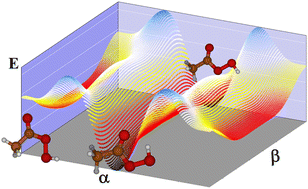
Peroxyacetic acid, a non-rigid oxygenated organic molecule which acts in the atmosphere as a reservoir of HOX and ROX radicals, is studied using highly correlated ab initio methods with the aim of its spectroscopic characterization in the gas phase. The study focuses on the far infrared region providing reliable rovibrational parameters such as energy levels and splittings. The molecule presents three conformers that inter-convert by internal rotation, drawing a potential energy surface of 12 minima. One of them shows prominent stability due to the formation of one weak intramolecular bond between the hydrogen atom of the hydroperoxy group and the oxygen atom of the carbonyl group. For the three minimum energy structures, rotational constants and centrifugal distortion constants are provided. It may be expected that the most stable conformer is the only one contributing to the spectral features in further measurements at low temperature. In this structure, the methyl torsional barrier has been found to be very low, V3 = 88.6 cm−1 producing a splitting of 2.262 cm−1 for the ground vibrational state. The study confirms that the ν20 torsional mode interacts strongly with the other two torsional modes ν19 and ν21, but slightly with the remaining vibrations. Then, a variational procedure in three dimensions allows the exploration of the low-frequency modes. The methyl torsional fundamental ν21 was found to be 49.1 cm−1 (Ai) and 33.4 cm−1 (E). The fundamentals of ν20 (C–O bond torsion) and ν19 (OH torsion) have been computed to be 216.7 cm−1 (A2) and 218.5 cm−1 (E) and 393.6 cm−1 (A2) and 394.1 cm−1. Since non-rigidity can have effects on the reactivity due to the conformer interconversion, and transitions involving low-lying levels can be observed with many spectroscopic techniques, this work can help kinetic studies and assignments of further spectroscopic studies needed for the detection in the gas phase of trace molecules.


 Please wait while we load your content...
Please wait while we load your content...