Bond-alternated and bond-equalized hexazine derivatives†
Abstract
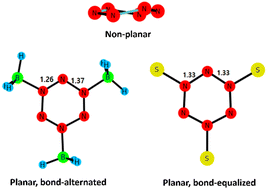
Planar cyclic conjugated molecules satisfying the Hückel rule are generally classified as aromatics and π delocalization is the key feature of aromatic compounds. It was shown that the π system of benzene prefers bond-alternation and that the delocalization observed is a consequence of bond-equalization, which is a σ effect. For systems wherein the π bonds are strong, such as those between N atoms, the π-distortivity may outweigh the σ preference for bond-equalization. Thus, one anticipates a bond-alternated structure for N6; however, neither the bond-equalized structure (D6h) nor the bond-alternated one (D3h) is a minimum as evident from the previous literature; calculations have shown that cyclic N6 is non-planar. Herein we show that a Lewis acid coordination strategy can be employed to stabilize the planar structure of N6. We show that the structure can be bond-alternated or bond-equalized depending on the strength of the Lewis acid. Kinetic stability with respect to concerted decomposition to three N–N triple bonded systems was assessed, and a few bond-equalized N6 systems were found to be potential candidates for synthesis.


 Please wait while we load your content...
Please wait while we load your content...