Azaindolizine proton cranes attached to 7-hydroxyquinoline and 3-hydroxypyridine: a comparative theoretical study†
Abstract
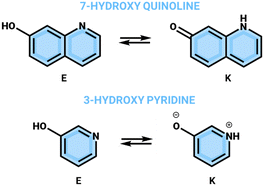
Theoretical design of several proton cranes, based on 7-hydroxyquinoline and 3-hydroxypyridine as proton-transfer frames, has been attempted using ground and excited-state density functional theory (DFT) calculations in various environments. Imidazo[1,2-a]pyridine, pyrazolo[1,5-a]pyridine and benzimidazole were considered as proton crane units. The proton crane action requires the existence of a single enol-like form in the ground state, which under excitation goes to the end keto-like one through a series of consecutive excited-state intramolecular proton transfers (ESIPT) and twisting steps with the participation of a crane unit, resulting in a long-range intramolecular proton transfer. The results suggest that 3-hydroxypyridine is not suitable for a proton-transfer frame and 8-(imidazo[1,2-a]pyridin-2-yl)quinolin-7-ol and 8-(pyrazolo[1,5-a]pyridin-2-yl)quinolin-7-ol behave as non-conjugated proton cranes, instead of tautomeric re-arrangement in the latter, which was thought to be possible.


 Please wait while we load your content...
Please wait while we load your content...