Modulation of Aβ 16–22 aggregation by glucose†
Abstract
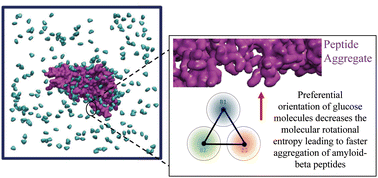
The self-assembly of amyloid-beta (Aβ) peptides into fibrillar structures in the brain is a signature of Alzheimer's disease. Recent studies have reported correlations between Alzheimer's disease and type-2 diabetes. Structurally, hyperglycemia induces covalent protein crosslinkings by advanced glycation end products (AGE), which can affect the stability of Aβ oligomers. In this work, we leverage physics-based coarse-grained molecular simulations to probe alternate thermodynamic pathways that affect peptide aggregation propensities at varying concentrations of glucose molecules. Similar to previous experimental reports, our simulations show a glucose concentration-dependent increase in Aβ aggregation rates, without changes in the overall secondary structure content. We discovered that glucose molecules prefer partitioning onto the aggregate–water interface at a specific orientation, resulting in a loss of molecular rotational entropy. This effectively hastens the aggregation rates, as peptide self-assembly can reduce the available surface area for peptide–glucose interactions. This work introduces a new thermodynamic-driven pathway, beyond chemical cross-linking, that can modulate Aβ aggregation.


 Please wait while we load your content...
Please wait while we load your content...