Selective crystal growth of magnesium hydroxide via solvent control for dye adsorption†
Abstract
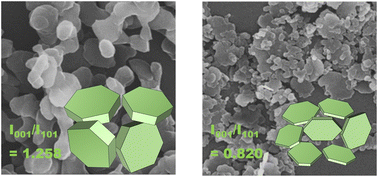
Magnesium hydroxide is an environmentally friendly and efficient material for the removal of harmful constituents from water by adsorption. The crystal facets and morphology have an important influence on the adsorption performance, thus the controllable growth of Mg(OH)2 and modification of its adsorption capacity has been a research focus. Herein, we report a solvent control method for synthesizing Mg(OH)2 with different polarities. By introducing ethanol and ethylene glycol in the crystallization solvent, the growth of crystal facets can be selectively promoted or inhibited. Besides, the adsorption of Congo red increases with the increase in the proportion of the (101) crystal facet, and Mg(OH)2 with large polarity and adsorption capacity can be obtained. Adsorption thermodynamics and kinetics show that the adsorption process is more consistent with the Langmuir isothermal adsorption model and quasi-second-order kinetic model. The removal rate of Congo red could reach 96%, and the maximum adsorption capacity reached 116.414 mg g−1.


 Please wait while we load your content...
Please wait while we load your content...