Development of actin dimerization inducers inspired by actin-depolymerizing macrolides†
Abstract
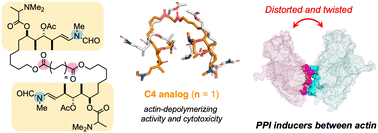
Several natural cytotoxic C2-symmetric bis-lactones, such as swinholide A and rhizopodin, sequester actin dimer from the actin network and potently inhibit actin dynamics. To develop new protein–protein interaction (PPI) modulators, we synthesized structurally simplified actin-binding side-chain dimers of antitumor macrolide aplyronine A. By fixing the two side-chains closer than those of rhizopodin, the C4 linker analog depolymerized filamentous actin more potently than natural aplyronines. Cross-link experiments revealed that actin dimer was formed by treatment with the C4 linker analog. Molecular dynamics simulations showed that this analog significantly changed the interaction and spatial arrangement of the two actins compared to those in rhizopodin to provide a highly distorted and twisted orientation in the complex. Our study may promote the development of PPI-based anticancer and other drug leads related to cytoskeletal dynamics.


 Please wait while we load your content...
Please wait while we load your content...