Micro-flow heteroatom alkylation via TfOH-mediated rapid in situ generation of carbocations and subsequent nucleophile addition†
Abstract
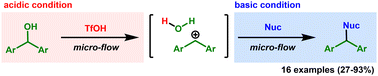
A rapid nucleophilic substitution reaction was developed using carbocations generated from diarylmethanol and trifluoromethanesulfonic acid. Undesired reactions caused by the carbocations were suppressed, presumably due to the rapid and uniform generation of carbocations and the subsequent rapid and uniform distribution of nucleophiles by the micro-flow technology.


 Please wait while we load your content...
Please wait while we load your content...