Asymmetric 1,2-diaxial synthesis of bi-(hetero)aryl benzofulvene atropisomers via transient directing group-assisted dehydrogenative coupling†
Abstract
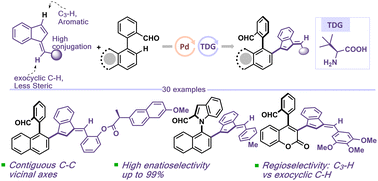
The efficient cross-dehydrogenative coupling of electronically rich and sterically congested benzofulvene with bi-(hetero)aryl moieties to construct an axially chiral benzofulvene core remains a formidable task. In this study, we describe a highly efficient and practical palladium-catalyzed approach for atroposelective bi-(hetero)aryl benzofulvene synthesis, achieving excellent enantioselectivity with moderate yields. This protocol offers a remarkable opportunity for the direct regio- and enantioselective conversion of C–H bonds of benzofulvene to C–C bonds. Furthermore, the protocol permits the incorporation of benzofulvene with a 4-phenyl coumarin core, enabling access to a novel class of axially chiral coumarins.


 Please wait while we load your content...
Please wait while we load your content...