Engineering cell-free systems by chemoproteomic-assisted phenotypic screening†
Abstract
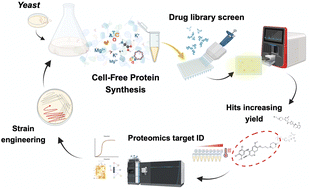
Phenotypic screening is a valuable tool to both understand and engineer complex biological systems. We demonstrate the functionality of this approach in the development of cell-free protein synthesis (CFPS) technology. Phenotypic screening identified numerous compounds that enhanced protein production in yeast lysate CFPS reactions. Notably, many of these were competitive ATP kinase inhibitors, with the exploitation of their inherent substrate promiscuity redirecting ATP flux towards heterologous protein expression. Chemoproteomic-guided strain engineering partially phenocopied drug effects, with a 30% increase in protein yield observed upon deletion of the ATP-consuming SSA1 component of the HSP70 chaperone. Moreover, drug-mediated metabolic rewiring coupled with template optimization generated the highest protein yields in yeast CFPS to date using a hitherto less efficient, but more cost-effective glucose energy regeneration system. Our approach highlights the utility of target-agnostic phenotypic screening and target identification to deconvolute cell-lysate complexity, adding to the expanding repertoire of strategies for improving CFPS.


 Please wait while we load your content...
Please wait while we load your content...