A novel lateral flow immunochromatographic assay using a recombinant VP2 antigen for total antibody detection of canine parvovirus-2†
Abstract
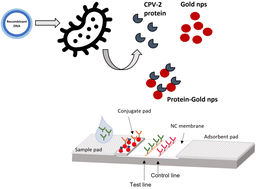
Canine parvovirus-2 (CPV-2) is a viral disease of dogs causing acute hemorrhagic gastroenteritis and myocarditis with high morbidity and mortality rates. The infection is still widespread all over the world. Vaccines developed against infection have great importance in preventing infection. However, it is difficult to recommend a practical vaccination program without knowing the antibody level of a puppy. Despite widespread vaccination, difficulties in detecting the maternal antibodies in puppies remain the main cause of vaccination failure. The hemagglutination inhibition (HAI) test is the gold standard to determine the immune status of dogs for canine parvovirus 2, but the HAI test has several disadvantages such as the need for fresh porcine blood, well-equipped laboratory, and long incubation periods. In this study, for the first time we developed a colloidal gold-based competitive lateral flow assay (cLFA) system for the rapid detection of total antibodies in canine serum using CPV-2b-VP2 derived from field isolates. The recombinantly expressed capsid protein of CPV-2 in the prokaryotic expression system was used as a labeled molecule in cLFA. We carried out studies on our cLFA system using the standard antibody solution and the clinical samples from vaccinated puppy serum. We compared the results of the LFAs with the HAI test. Competitive lateral flow assay results showed good correlation with the gold standard method, the HAI test. In the developed platform, the limit of detection of the standard antibody was determined to be 375 ng mL−1, while the cut-off level of antibodies was observed to be 1 : 40 HAI titer in clinical samples. Our reported system will be a strong alternative for CPV-2 antibody-based detection applications.


 Please wait while we load your content...
Please wait while we load your content...