Complexation of gallic acid with calcium: electrochemical, potentiometric, and UV-VIS studies
Abstract
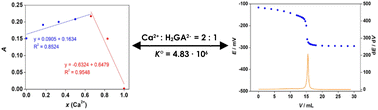
Gallic acid and its metal complexes are good antioxidants that could be used to increase the body's immune system against degenerative and viral diseases. Therefore, such complexes could be used as a good alternative to supplementary medicines and may have potential significance in clinical trials. Differential pulse voltammetry, UV/VIS spectroscopy, and potentiometry were used to analyse the complexation of gallic acid with calcium in this study. The metal : ligand ratio was determined using Job's continuous variation method and was found to be 2 : 1. The stability constant of calcium gallate was determined with potentiometric titration with a calcium electrode, which amounted to K° = 4.83 × 106 (log K° = 6.7). The combination of the results obtained in this study gives a deeper insight into the complexation of gallic acid with calcium and determines the stability constant of calcium gallate.


 Please wait while we load your content...
Please wait while we load your content...