J-Aggregation induced NIR-II fluorescence: an aza-BODIPY luminogen for efficient phototheranostics†
Abstract
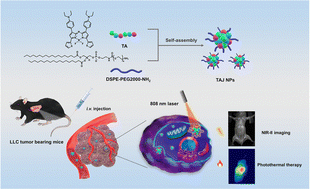
The development of organic dyes with emission peaks in the second near-infrared window (NIR-II 1000–1700 nm) is highly desirable for in vivo imaging and imaging-guided phototheranostics. However, the lack of appropriate molecular frameworks and the challenges associated with complex synthesis critically hinder the development of new candidate fluorophores. J-Aggregation is considered as a smart and straightforward way to construct such a therapeutic agent with NIR-II fluorescence imaging properties. Here, we present the design and synthesis of an aza-BODIPY probe (TA). Upon encapsulation within the amphiphilic polymer DSPEG-PEG2000-NH2, TA underwent self-assembly and formed J-aggregates (TAJ NPs), which showed emission at 1020 nm. High spatial resolution and adequate signal-to-noise ratio of the TAJ NPs are demonstrated for noninvasive bioimaging of the vasculature, lymph nodes and bones of mice in the NIR-II region. Moreover, the TAJ NPs exhibited good tumor enrichment efficiency with reduced liver accumulation and significant imaging-guided phototherapy performance against lung cancer cells. Taken together, this work not only introduces a new NIR-II imaging and phototheranostic agent based on J-aggregates, but also provides insight into the development of versatile organic dyes for future clinical implementation.


 Please wait while we load your content...
Please wait while we load your content...