Development of tris(amino)phosphonium electrolytes for high performing sodium batteries†
Abstract
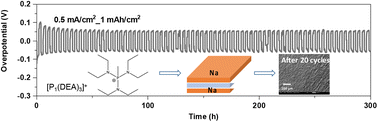
Sodium batteries are promising alternatives to state-of-the-art lithium batteries, owing to the cheaper resources and more sustainable materials. To realise this promise, the requirement for electrochemically and thermally stable electrolytes with low volatility and flammability to enable high-performance sodium batteries is crucial. In this work, we reported a unique family of salts through the synthesis of tris(amino)phosphonium cations [P1(DEA)3]+ and pairing with [FSI]− and [TFSI]− anions. The unique organic ionic plastic crystal (OIPC) [P1(DEA)3][FSI] displays high ionic conductivity which is several orders of magnitude greater than that of its counterpart [P1(DMA)3][FSI]. Electrolytes composed of 50 mol% NaFSI in [P1(DEA)3][FSI] showed a high conductivity value of 1.6 × 10−2 S cm−1 at 50 °C. The electrolyte supports stable Na plating/stripping with a small overpotential of 70 mV at 0.5 mA cm−2 with 2 h polarization. This electrolyte exhibits a satisfactory performance in sodium metal full cells (Na metal and NaFePO4 electrodes) with a stable cycling performance of 125 mA h g−1 at C/10, a high capacity retention of ∼80% after 112 cycles and a coulombic efficiency close to 99.5%.
- This article is part of the themed collection: Celebrating the scientific accomplishments of RSC Fellows


 Please wait while we load your content...
Please wait while we load your content...