Mechanistic insights into trisulfur radical generation in lithium–sulfur batteries†
Abstract
Trisulfur radicals (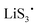 and
and 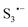 ) are crucial reaction intermediates that can activate new reaction pathways to facilitate sulfur species conversion in liquid electrolytes of lithium–sulfur batteries. Understanding the complicated solution chemistry of trisulfur radical generation can help to discover new strategies for improving sulfur conversion kinetics. In this work, we employ density functional theory (DFT) to investigate the generation mechanism of trisulfur radicals in dimethyl sulfoxide (DMSO) and 1,3-dioxolane (DOL) solvents. DMSO solvent with a higher dielectric constant and a larger donor number has a stronger lithiophilicity than DOL and can weaken the Li–S bonds of Li-containing polysulfides more efficiently, resulting in the discrepancy that Li2S6 can dissociate into
) are crucial reaction intermediates that can activate new reaction pathways to facilitate sulfur species conversion in liquid electrolytes of lithium–sulfur batteries. Understanding the complicated solution chemistry of trisulfur radical generation can help to discover new strategies for improving sulfur conversion kinetics. In this work, we employ density functional theory (DFT) to investigate the generation mechanism of trisulfur radicals in dimethyl sulfoxide (DMSO) and 1,3-dioxolane (DOL) solvents. DMSO solvent with a higher dielectric constant and a larger donor number has a stronger lithiophilicity than DOL and can weaken the Li–S bonds of Li-containing polysulfides more efficiently, resulting in the discrepancy that Li2S6 can dissociate into 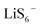 and
and 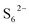 in DMSO, while keeping a neutral state in DOL. The computed reaction energetics suggest that hexasulfides (Li2S6,
in DMSO, while keeping a neutral state in DOL. The computed reaction energetics suggest that hexasulfides (Li2S6, 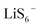 , and
, and 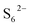 ) are thermodynamically feasible to generate trisulfur radicals in DMSO, and the generation reaction would be more exergonic when hexasulfides are more fully solvated. However, the formation of trisulfur radicals in DOL is always unfavorable. Possible reaction mechanisms to produce trisulfur radicals are further proposed, and the computed maximum free energy barriers along the proposed pathways are 9.62 and 19.08 kcal mol−1 in DMSO and DOL, respectively, that explains the solvent-dependence of trisulfur radical generation from the kinetic aspect. This work provides a deeper understanding of solution-based sulfur chemistry and contributes to electrolyte optimization for high-rate polysulfide conversion.
) are thermodynamically feasible to generate trisulfur radicals in DMSO, and the generation reaction would be more exergonic when hexasulfides are more fully solvated. However, the formation of trisulfur radicals in DOL is always unfavorable. Possible reaction mechanisms to produce trisulfur radicals are further proposed, and the computed maximum free energy barriers along the proposed pathways are 9.62 and 19.08 kcal mol−1 in DMSO and DOL, respectively, that explains the solvent-dependence of trisulfur radical generation from the kinetic aspect. This work provides a deeper understanding of solution-based sulfur chemistry and contributes to electrolyte optimization for high-rate polysulfide conversion.



 Please wait while we load your content...
Please wait while we load your content...