Electron transfer driving force as the criterion for efficient n-doping of organic semiconductors with DMBI-H derivatives†
Abstract
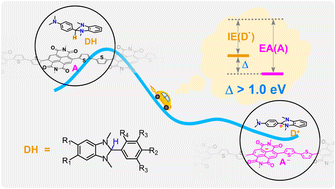
Restricted by the low doping efficiency, the conductivity of n-type organic semiconductors (OSCs) lags far behind their p-type counterparts. DMBI-H derivatives are the most widely applied n-dopants, and the hydride transfer (HYT) to OSCs has been recognized as the most possible rate-determining step in the n-doping reaction. However, relations of the doping efficiency to molecular properties of OSCs and dopants remain unclear. Here, by calculating the thermodynamics and kinetics of doping reactions between eight DMBI-H derivatives and 52 OSCs using the density functional theory method, the electron affinity (EA) of OSCs and the ionization energy (IE) of dopant radicals (D˙s) are identified as molecular descriptors of the n-doping reaction. The EA(OSC) − IE(D˙) > 1.0 eV criterion is proposed to assess the doping efficiency of these DMBI-H derivatives. The hydrogen release and possible side-products are also discussed in relation to the backbone structure of OSCs. Finally, these concepts are proved using four newly designed n-type polymers with EAs > 4.0 eV. The descriptors and criterion not only can be used to predict viable n-dopants and doping conditions for known OSCs, but also provide a rule of thumb for the molecular design of new OSCs and dopants.


 Please wait while we load your content...
Please wait while we load your content...