High metal loaded Cu(i)N3 single-atom catalysts: superior methane conversion activity and selectivity under mild conditions†
Abstract
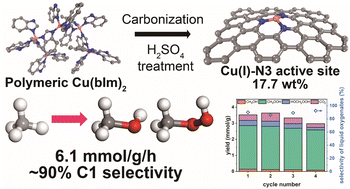
Direct conversion of methane to liquefied products, such as methanol, is an essential process for the effective use of natural gas resources during storage and transportation. However, this process remains a challenge due to the high energy barrier of the C–H bond. To address this issue, we have developed a N-doped carbon catalyst (CuNC-600), containing under-coordinated single-atom Cu(I)N3 active centers with an ultra-high metal loading of up to 17.7 wt%. Our study reports a mass activity of 6.1 mmol g−1 at 50 °C using H2O2 as an oxidant, which outperforms the previously reported thermocatalysts including not only non-noble metals (Cu and Fe), but also noble metals (AuPd and Rh), with a comparable selectivity of ∼90% toward C1 liquid products. This considerable methane conversion activity was achieved by combining both high metal loading and the under-coordinated Cu active site. The experimental studies revealed that the superoxide radical governs the overall reaction pathways of CH3OH and CH3OOH production, while the hydroxyl radical is mainly involved in methane activation.


 Please wait while we load your content...
Please wait while we load your content...