In situ ionothermally synthesized redox-active carbon nitride-confined organic small molecule cathodes for ultrastable lithium-ion batteries†
Abstract
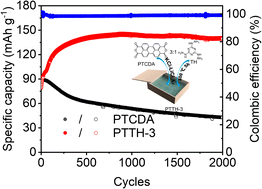
Despite their high specific capacity and low cost, small-molecule organic cathodes usually suffer from fast capacity decay due to unavoidable dissolution in electrolytes, which largely impedes their practical applications. To resolve the above-mentioned issues, an in situ ionothermal synthesis strategy is proposed to prepare a C3N5 polymer matrix with azo groups using 2,4,6-tris(hydrazino)-s-triazine (TH) as the precursor for confining active small molecules to enhance the electrochemical stability. Herein, perylene-3,4,9,10-tetracarboxylic dianhydride (PTCDA) and 1,4,5,8-naphthalenetetracarboxylic dianhydride (NTCDA) are used as exemplary molecules to demonstrate this strategy. Consequently, among optimized composites, PTTH-3 delivers the highest reversible capacity of 170 mA h g−1 at 0.1 A g−1, best rate capability (142 mA h g−1 at 1 A g−1 and 83 mA h g−1 at 20 A g−1) and the most excellent cycling stability with a capacity retention of 97.5% after 2000 cycles at 1 A g−1, better than all previously reported PTCDA cathodes. Additionally, NTTH-3 also shows far better electrochemical properties with a capacity retention of 84% after 2200 cycles at 1 A g−1 than NTCDA alone, indicative of the universality of this strategy. This method may pave the way for the practical application of high-capacity small molecular compounds in electrochemical energy storage.


 Please wait while we load your content...
Please wait while we load your content...