Efficient urea formation from N2O + CO on dual-atom catalysts TM2/g-CN†
Abstract
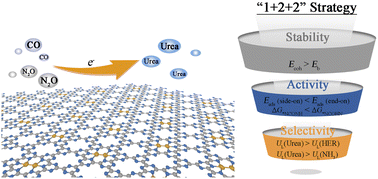
In industry, urea is synthesized under harsh conditions, which consumes approximately 80% of the global NH3. Therefore, it is important to develop electrocatalytic technology for green and sustainable urea synthesis. In this work, we propose a new mechanism for electrocatalytic urea synthesis, with N2O and CO as the nitrogen and carbon sources, respectively. In accordance with a “1 + 2 + 2” strategy, dual-atom catalysts (DACs) TM2/g-CN with high stability, activity and selectivity for urea formation are screened. The results indicate that Cr2/g-CN and Co2/g-CN are highly active toward electrocatalytic urea formation with rather low limiting potentials, −0.19 and −0.15 V, respectively. At the same time, the competitive nitrogen reduction reaction (NRR) and hydrogen evolution reaction (HER) can be effectively suppressed. More interestingly, urea formation on Fe2/g-CN occurs spontaneously, due to the appropriate adsorption strength of intermediates. We identified that ΔG*NCON is a simple and effective descriptor to predict and design potential electrocatalysts toward electrocatalytic urea synthesis.


 Please wait while we load your content...
Please wait while we load your content...