A metal–organic framework modulated to site-isolate Cl˙ pendants via radical inter-conversion for degrading hard-to-ionize aqueous organic wastes†
Abstract
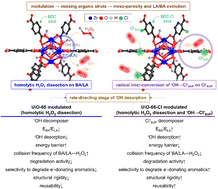
Compared with conventional ˙OH, Cl˙ is longer-lived, more selective to destabilizing refractory electron (e−)-donating aqueous aromatics via radicalization, and renewable via e− transfer from aromatics to enable Cl˙ ↔ Cl− inter-conversion. To demonstrate the merits of Cl˙, a Cl pendant (ClSUP)-functionalized Zr-based metal–organic framework (UiO-66-Cl) was synthesized/modulated to impart mesoporosity for facilitating the diffusion of bulky aromatics into the porous architecture. UiO-66-Cl could site-isolate Cl− anions (Cl−SUP) near Lewis acidic Zr4+ cations (LA) and Brønsted acidic –OH (BA), on which ˙OH was produced via homolytic H2O2 dissection, desorbed, and bound to Cl−SUP to yield Cl˙SUPvia exothermic radical inter-conversion of ˙OH → Cl˙SUP (referred to as the overall ˙OH → Cl˙SUP route). UiO-66-Cl provided greater LA/BA strengths than UiO-66 un-functionalized with ClSUP/Cl−SUP, thus requiring a lower energy for ˙OH desorption, which was identified as the rate-determining step of homolytic H2O2 dissection on UiO-66 or the overall ˙OH → Cl˙SUP route on UiO-66-Cl. Consequently, Cl˙SUP productivity on UiO-66-Cl was higher than ˙OH productivity on UiO-66 (activity↑). Moreover, UiO-66-Cl exploited Cl˙SUP as the major decomposer of e−-donating aromatics (selectivity↑) via the e− transfer pathway (recyclability↑), as proved by DFT calculations, EPR spectroscopy, and filtration/scavenging/isotope control runs. Furthermore, UiO-66-Cl was more resistant to structural deformation upon exposure to extreme reaction environments than UiO-66 (stability↑), as verified by DFT calculations/XRD analysis. Hence, UiO-66-Cl (Cl˙SUP) outperformed UiO-66 (˙OH), SO42−-functionalized iron oxide (SO4˙−SUP), or NO3−-modified Mn oxide (NO3˙SUP) in degrading e−-donating, ionization-resistant aqueous aromatics (phenol, aniline, acetaminophen, sulfanilamide, and sulfamethoxazole) in terms of activity, selectivity, stability, and/or reusability.


 Please wait while we load your content...
Please wait while we load your content...