Enhanced ionic conductivity of protonated antiperovskites via tuning lattice and rotational dynamics†
Abstract
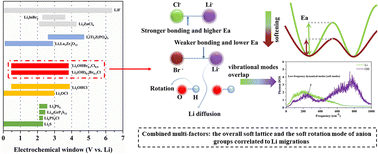
The thermodynamically more stable lithium halide hydroxide, Li2OHCl, is experimentally easier to synthesize than Li3OCl. However, the protonated antiperovskite has low ionic conductivity at room temperature due to the limited reorientation of OH anions. Here, density functional theory calculations were performed to determine the stability, elastic properties and Li diffusion of brominated Li2OHCl (Li2(OH)0.9Br0.1Cl and Li2OHCl0.9Br0.1). The Br substitution weakens the local bonding interactions and promotes the reorientation of OH anions, thus increasing the ionic conductivities. Based on this, the reorientation of OH anions is further accelerated by substituting Cl with BH4 anions, and this novel protonated antiperovskite exhibits a high ionic conductivity of 2.1 mS cm−1 at room temperature. Our work highlights that combining multiple factors, the overall soft lattice and the soft rotation mode of anion groups correlated to Li migrations, can effectively optimize the Li ion conductivity, which might be a universal descriptor to further screen or design other classes of solid-state electrolytes.


 Please wait while we load your content...
Please wait while we load your content...