Solvate ionic liquid-derived solid polymer electrolyte with lithium bis(oxalato) borate as a functional additive for solid-state lithium metal batteries†
Abstract
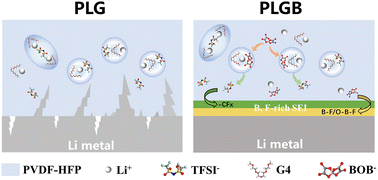
Solid-state lithium metal batteries have great application prospects due to their high theoretical energy density but are restricted by the low ionic conductivity of the solid electrolyte and unsatisfactory interface chemistry between the electrode and electrolyte. Herein, a novel solid polymer electrolyte (SPE) was obtained by incorporating a solvate ionic liquid (SIL) [Li(G4)1][TFSI] containing LiBOB as a functional additive into the polymer matrix. Profiting from the synergistic effect of the SIL and the Li+ conductive salt, the uniquely designed SPE has high ionic conductivity, wide electrochemical window, high Li+ transference number and excellent compatibility with metallic Li. Accordingly, it endows the solid-state lithium metal battery with superior long-term cyclability at room temperature. The addition of LiBOB not only improves the Li+ coordination and conductive environment, but also contributes to the rigid-flexible coupling interface chemistry to buffer the volume change in Li metal upon cycling and achieve homogeneous and dendrite-free Li deposition.
- This article is part of the themed collection: Editor’s choice: Li-metal batteries


 Please wait while we load your content...
Please wait while we load your content...