Boosting electrocatalytic water oxidation of NiFe layered double hydroxide via the synergy of 3d–4f electron interaction and citrate intercalation†
Abstract
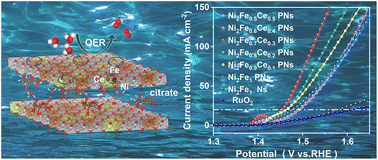
Rational morphology design and electronic structure modulation can facilely boost the oxygen evolution reaction (OER) of NiFe layered double hydroxides (LDHs). Herein, hierarchical trimetallic NiFeCe-LDH intercalated with citrate anions was synthesized via a co-precipitation method. The self-supported porous LDH nanosheets (PNs) with abundant active sites exposed contributed to robust structural stability while the intercalated citrate and the introduction of Ce ions optimized intrinsic activity by modulating the electronic structure through 3d–4f electron interaction. As a result, PNs with an optimized Ni : Fe : Ce molar ratio of 2 : 0.7 : 0.3 exhibited the best OER activity of delivering 20 mA cm−2 at an overpotential of only 224 mV with a Tafel slope of 54.0 mV dec−1 and excellent durability of ∼160 h at a high current density of 100 mA cm−2. Experimental and theoretical investigations further confirmed the strong 3d–4f electron interaction, dramatically improving the adsorption/desorption properties of the oxygen-containing intermediates. This work provides a strategy to develop high-performance electrocatalysts through the rational synergism of 3d–4f electron interaction and appropriate anion composition.


 Please wait while we load your content...
Please wait while we load your content...