Improved 2-pyridyl reductive homocoupling reaction using biorenewable solvent Cyrene™ (dihydrolevoglucosenone)†
Abstract
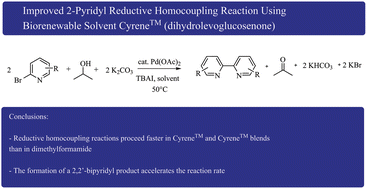
The synthesis of 5,5′-bis(trifluoromethyl)-2,2′-bipyridine using 2-bromo-5-(trifluoromethyl) pyridine was achieved at 50 °C using palladium acetate, tetrabutylammonium iodide (TBAI), potassium carbonate, and isopropanol in Cyrene™ (dihydrolevoglucosenone), a bio-renewable “green” solvent formed by a two-step process from cellulose. Improvements were achieved with 50% of γ-valerolactone (GVL) in Cyrene™ resulting in a 95% yield and 99% product purity without the use of column chromatography or recrystallization. At 80 °C, the reaction was completed within 1 h. Full conversion with 1 mol% instead of 15 mol% of palladium acetate was observed within 10 h. We showed that the formed 2,2′-bipyridine product significantly accelerated the reaction probably due to the stabilization of the catalytic species. The addition of TBAI was essential for the rapid homocoupling, however, 20 mol% of TBAI was sufficient to reach full conversion of 2-bromo-5-(trifluoromethyl) pyridine within 6 h at 80 °C. Another improvement was observed with the substitution of isopropanol by 1,4-butanediol achieving full conversion within 6 h. 2-Bromopyridines with electron withdrawing substituents in the 6, 5, 4 ring position reacted under these conditions. 2-Bromopyridines with an electron donating substituent reacted slower. Overall, we demonstrated that the 50% GVL in Cyrene™ blend is a superior “green” and less toxic alternative to dimethylformamide for the reductive homocoupling reaction. Using a quantitative scoring for twelve principles of green chemistry (DOZN™), we found significant improvements that were mediated by higher yield (atom economy), shorter heating time and lower reaction temperature (energy efficiency), safer solvent (hazardous chemical synthesis), and safer chemistry (accident prevention).


 Please wait while we load your content...
Please wait while we load your content...