Understanding the different deoxygenation reaction pathways of lauric acid over alumina-supported Ni and Co catalysts†
Abstract
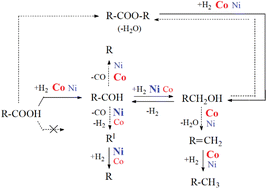
Deoxygenation of aliphatic acids into hydrocarbons is essential to produce sustainable aviation fuel (SAF) components. Depending on the catalyst, different reaction pathways are preferred, determining thus the deoxygenation efficiency and yields and selectivity of the desired hydrocarbons. Despite being extensively studied, the striking difference in the performance of Ni and Co catalysts, both being very promising deoxygenation catalysts, has not yet been explained. As this is of utmost importance for the rational design of deoxygenation catalysts, we used lauric acid as a model compound and focused not only on its reactivity but also on that of its reaction intermediates (lauric aldehyde, lauryl alcohol and lauryl laurate). We used an alumina support that we impregnated with the same amount of either Ni or Co, which allowed us to investigate the intrinsic differences between both metals. The prepared catalysts were characterized by XRD, H2-TPR chemisorption and N2 physisorption methods. All catalytic experiments were carried out in a Parr stirred autoclave at T = 280 °C and p = 40 bar under either a H2 or N2 atmosphere. No conversion of the acid was observed in N2 for both catalysts, proving that the initial hydrogenation of the acid to the corresponding aldehyde was essential. This hydrogenation step, as well as the hydroconversion of lauryl laurate formed by the interaction of lauric acid with lauryl alcohol, was more efficient on Co/Al2O3, while Ni/Al2O3 was more active in hydrogenating lauric aldehyde to lauryl alcohol and decarbonylating the aldehyde to C11 hydrocarbon. Experiments under a N2 atmosphere also revealed differences in the routes of lauric aldehyde decarbonylation. In the case of Co/Al2O3, C11 alkane was formed by the direct decarbonylation of the aldehyde, while the decarbonylation route occurred via the formation of a ketene intermediate over Ni/Al2O3, resulting, thus, in the formation of C11 olefins. We calculated the initial consumption rates of the reactants and the rates of hydrocarbon formation, which allowed us to propose a comparative reaction network for lauric acid deoxygenation over Ni/Al2O3 and Co/Al2O3. This will facilitate the design of effective deoxygenation catalysts for aliphatic acid valorization into SAF components.


 Please wait while we load your content...
Please wait while we load your content...