Modular preparation of cationic bipyridines and azaarenes via C–H activation†
Abstract
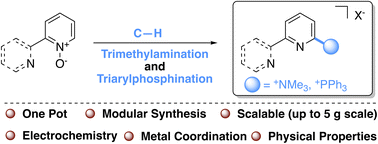
Bipyridines are ubiquitous in organic and inorganic chemistry because of their redox and photochemical properties and their utility as ligands to transition metals. Cationic substituents on bipyridines and azaarenes are valuable as powerful electron-withdrawing functionalities that also enhance solubility in polar solvents, but there are no general methods for direct functionalization. A versatile method for the preparation of trimethylammonium- and triarylphosphonium-substituted bipyridines and azaheterocycles is disclosed. This methodology showcases a C–H activation of pyridine N-oxides that enables a highly modular and scalable synthesis of a diverse array of cationically charged azaarenes. The addition of trimethylammonium functionalities on bipyridine derivatives resulted in more anodic reduction potentials (up to 700 mV) and increased electrochemical reversibility compared to the neutral unfunctionalized bipyridine. Additonally, metallation of 4-triphenylphosphinated biquinoline to make the corresponding Re(CO)3Cl complex resulted in reduction potentials 400 mV more anodic than the neutral derivative.


 Please wait while we load your content...
Please wait while we load your content...