Enhancing organic cathodes of aqueous zinc-ion batteries via utilizing steric hindrance and electron cloud equalization†
Abstract
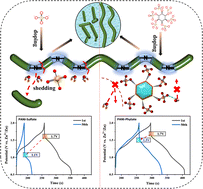
Polyaniline (PANI), with merits of high electronic conductivity and capacity, is a promising material for zinc (Zn)-ion batteries. However, its redox window in Zn batteries is often limited, mainly due to the oxidative degradation at high potentials—in which imine groups can be attacked by water molecules. Here, we introduce phytic acid, a kind of supermolecule acid radical ion, as a dopant and electrolyte additive. Various in/ex situ analyses and theoretical calculations prove that the steric hindrance effect can prevent electroactive sites from the attack by water molecules. Meanwhile, the redox reaction can be stabilized by an even distribution of electron cloud due to the conjugated structure of phenazine groups. Accordingly, the assembled Zn–PANI battery can allow stable and long-term charge–discharge reactions to occur at a potential as high as 2.0 V with a discharged plateau of 1.5 V, and it also shows high rate performance and stable long cycle life (75% capacity retention after 1000 cycles at 10 A g−1).
- This article is part of the themed collection: Most popular 2023 energy & environmental chemistry articles


 Please wait while we load your content...
Please wait while we load your content...