Photocatalytic redox-neutral selective single C(sp3)–F bond activation of perfluoroalkyl iminosulfides with alkenes and water†
Abstract
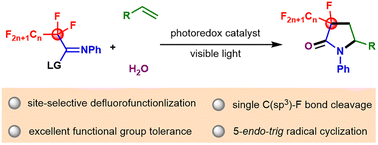
Visible-light-promoted site-selective and direct C–F bond functionalization of polyfluorinated iminosulfides was accomplished with alkenes and water under redox-neutral conditions, affording a diverse array of γ-lactams with a fluoro- and perfluoroalkyl-substituted carbon centre. A variety of perfluoroalkyl units, including C2F5, C3F7, C4F9, and C5F11 underwent site-selective defluorofunctionalization. This protocol allows high chemoselectivity control and shows excellent functional group tolerance. Mechanistic studies reveal that the remarkable changes of the electron geometries during the defluorination widen the redox window between the substrates and the products and ensure the chemoselectivity of single C(sp3)–F bond cleavage.
- This article is part of the themed collection: Most popular 2023 organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...