Site selective gold(i)-catalysed benzylic C–H amination via an intermolecular hydride transfer to triazolinediones†
Abstract
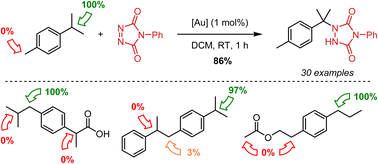
Triazolinediones are known as highly reactive dienophiles that can also act as electrophilic amination reagents towards enolisable C–H bonds (ionic pathway) or weak C–H bonds (free radical pathway). Here, we report that this C–H amination reactivity can be significantly extended and enhanced via gold(I)-catalysis. Under mild conditions, several alkyl-substituted aryls successfully undergo benzylic C–H aminations at room temperature. The remarkable site selectivity that is observed points towards strong electronic activation and deactivation effects, that go beyond a simple weakening of the C–H bond. The observed catalytic C–H aminations do not follow the expected trends for a free radical-type C–H amination and show complementarity to existing methods. Density functional theory (DFT) calculations and distinct experimental trends provide a clear mechanistic rationale for observed selectivity patterns, postulating a novel pathway for triazolinedione-induced aminations via a carbon-to-nitrogen hydride transfer.


 Please wait while we load your content...
Please wait while we load your content...