Photochemical charge accumulation in a heteroleptic copper(i)-anthraquinone molecular dyad via proton-coupled electron transfer†
Abstract
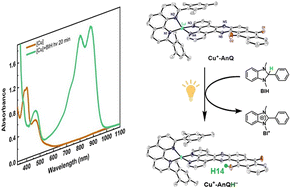
Developing efficient photocatalysts that perform multi electron redox reactions is critical to achieving solar energy conversion. One can reach this goal by developing systems which mimic natural photosynthesis and exploit strategies such as proton-coupled electron transfer (PCET) to achieve photochemical charge accumulation. We report herein a heteroleptic Cu(I)bis(phenanthroline) complex, Cu-AnQ, featuring a fused phenazine-anthraquinone moiety that photochemically accumulates two electrons in the anthraquinone unit via PCET. Full spectroscopic and electrochemical analyses allowed us to identify the reduced species and revealed that up to three electrons can be accumulated in the phenazine-anthraquinone ring system under electrochemical conditions. Continuous photolysis of Cu-AnQ in the presence of sacrificial electron donor produced doubly reduced monoprotonated photoproduct confirmed unambiguously by X-ray crystallography. Formation of this photoproduct indicates that a PCET process occurred during illumination and two electrons were accumulated in the system. The role of the heteroleptic Cu(I)bis(phenanthroline) moiety participating in the photochemical charge accumulation as a light absorber was evidenced by comparing the photolysis of Cu-AnQ and the free AnQ ligand with less reductive triethylamine as a sacrificial electron donor, in which photogenerated doubly reduced species was observed with Cu-AnQ, but not with the free ligand. The thermodynamic properties of Cu-AnQ were examined by DFT which mapped the probable reaction pathway for photochemical charge accumulation and the capacity for solar energy stored in the process. This study presents a unique system built on earth-abundant transition metal complex to store electrons, and tune the storage of solar energy by the degree of protonation of the electron acceptor.
- This article is part of the themed collection: Most popular 2023 catalysis articles


 Please wait while we load your content...
Please wait while we load your content...