Rhodium(i)-catalyzed cascade C(sp2)–H bond alkylation – amidation of anilines: phosphorus as traceless directing group†
Abstract
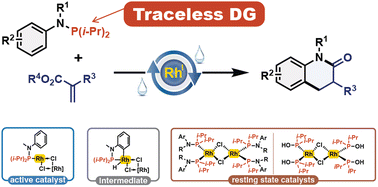
We introduce a versatile Rh(I)-catalyzed cascade reaction, combining C(sp2)–H bond functionalization and amidation between N-arylphosphanamines and acrylates. This innovative approach enables the rapid synthesis of dihydroquinolinone scaffolds, a common heterocycle found in various pharmaceuticals. Notably, the presence of the phosphorus atom facilitates the aniline ortho-C(sp2)–H bond activation prior to N–P bond hydrolysis, streamlining one-pot intramolecular amidation. Moreover, we demonstrate the applicability of this reaction by synthesizing an antipsychotic drug. Detailed mechanistic investigations revealed the involvement of a Rh–H intermediate, with substrate inhibition through catalyst saturation.


 Please wait while we load your content...
Please wait while we load your content...