Zn-induced electron-rich Sn catalysts enable highly efficient CO2 electroreduction to formate†
Abstract
Renewable-energy-driven CO2 electroreduction provides a promising way to address the growing greenhouse effect issue and produce value-added chemicals. As one of the bulk chemicals, formic acid/formate has the highest revenue per mole of electrons among various products. However, the scaling up of CO2-to-formate for practical applications with high faradaic efficiency (FE) and current density is constrained by the difficulty of precisely reconciling the competing intermediates (*COOH and HCOO*). Herein, a Zn-induced electron-rich Sn electrocatalyst was reported for CO2-to-formate with high efficiency. The faradaic efficiency of formate (FEformate) could reach 96.6%, and FEformate > 90% was maintained at formate partial current density up to 625.4 mA cm−1. Detailed study indicated that catalyst reconstruction occurred during electrolysis. With appropriate electron accumulation, the electron-rich Sn catalyst could facilitate the adsorption and activation of CO2 molecules to form a 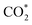 intermediate and then promoted the carbon protonation of
intermediate and then promoted the carbon protonation of 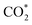 to yield a HCOO* intermediate. Afterwards, the HCOO* → HCOOH* proceeded via another proton-coupled electron transfer process, leading to high activity and selectivity for formate production.
to yield a HCOO* intermediate. Afterwards, the HCOO* → HCOOH* proceeded via another proton-coupled electron transfer process, leading to high activity and selectivity for formate production.



 Please wait while we load your content...
Please wait while we load your content...