Stereoselective two-carbon ring expansion of allylic amines via electronic control of palladium-promoted equilibria†
Abstract
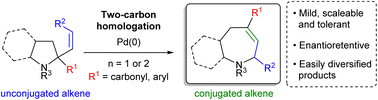
General methodologies enabling the two-carbon homologation of pyrrolidine and piperidine systems have yet to be developed. Herein we report that palladium-catalysed allylic amine rearrangements enable efficient two-carbon ring expansion of 2-alkenyl pyrrolidine and piperidines to their azepane and azocane counterparts. Conditions are mild, tolerant of a range of functional groups and the process can occur with high enantioretention. The products formed undergo a range of orthogonal transformations, making them ideal scaffolds for the creation of compound libraries.


 Please wait while we load your content...
Please wait while we load your content...