A dinuclear Rh(−i)/Rh(i) complex bridged by biphilic phosphinine ligands†
Abstract
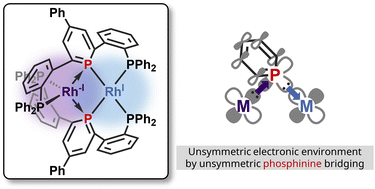
Bimetallic complexes have enabled precise control of catalysis by accumulating two discrete metal centres. In these complexes, bridging ligands are essential to combine multiple metals into one molecule. Among some bridging modes, an unsymmetric bridging mode will differentiate the electronic structures of the two metal centres. In this study, a dinuclear Rh(−I)/Rh(I) complex bridged by tridentate phosphine–phosphinine–phosphine ligands was prepared by reduction of the corresponding Rh(I) complex. Single-crystal X-ray analysis and DFT calculations suggest that the phosphinine ligands adopt an unsymmetric bridging mode wherein phosphinine accepts d-electrons from one Rh centre and, at the same time, donates lone pairs to the other Rh centre.


 Please wait while we load your content...
Please wait while we load your content...