Palladium mono-N-protected amino acid complexes: experimental validation of the ligand cooperation model in C–H activation†
Abstract
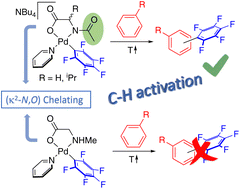
Mechanistic proposals for the C–H activation reaction enabled by mono-N-protected amino acid ligands (MPAAs) have been supported by DFT calculations. The direct experimental observation of the ligand-assisted C–H activation has not yet been reported due to the lack of well-defined isolated palladium complexes with MPAAs that can serve as models. In this work, palladium complexes bearing chelating MPAAs (NBu4)[Pd(κ2-N,O–AcN–CHR–COO)(C6F5)py] (Ac = MeC(O); R = H, Me) and [Pd(κ2-N,O–MeNH–CH2–COO)(C6F5)py] have been isolated and characterized. Their evolution in a solution containing toluene leads to the C–H activation of the arene and the formation of the C6F5–C6H4Me coupling products. This process takes place only for the ligands with an acyl protecting group, showing the cooperating role of this group in a complex with a chelating MPAA, therefore experimentally validating this working model. The carboxylate group is inefficient in this C–H activation.
- This article is part of the themed collection: #MyFirstChemSci 2023


 Please wait while we load your content...
Please wait while we load your content...