Sodium mediated deprotonative borylation of arenes using sterically demanding B(CH2SiMe3)3: unlocking polybasic behaviour and competing lateral borane sodiation†
Abstract
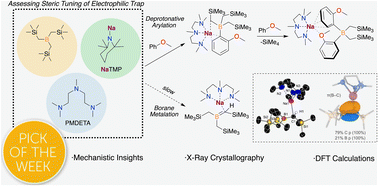
The deprotonative metalation of organic molecules has become a convenient route to prepare functionalised aromatic substrates. Amongst the different metallating reagents available, sodium bases have recently emerged as a more sustainable and powerful alternative to their lithium analogues. Here we report the study of the sterically demanding electrophilic trap B(CH2SiMe3)3 for the deprotonative borylation of arenes using NaTMP (TMP = 2,2,6,6-tetramethylpiperidide) in combination with tridentate Lewis donor PMDETA (PMDETA = N,N,N′,N′′,N′′-pentamethyldiethylenetriamine). Using anisole and benzene as model substrates, unexpected polybasic behaviour has been uncovered, which enables the formal borylation of two equivalents of the relevant arene. The combination of X-ray crystallographic and NMR monitoring studies with DFT calculations has revealed that while the first B–C bond forming process takes place via a sodiation/borylation sequence to furnish [(PMDETA)NaB(Ar)(CH2SiMe3)3] species, the second borylation step is facilitated by the formation of a borata-alkene intermediate, without the need of an external base. For non-activated benzene, it has also been found that under stoichimetric conditions the lateral sodiation of B(CH2SiMe3)3 becomes a competitive reaction pathway furnishing a novel borata-alkene complex. Showing a clear alkali-metal effect, the use of the sodium base is key to access this reactivity, while the metalation/borylation of the amine donor PMDETA is observed instead when LiTMP is used.
- This article is part of the themed collections: 2023 Chemical Science HOT Article Collection, #MyFirstChemSci 2023 and 2023 ChemSci Pick of the Week Collection


 Please wait while we load your content...
Please wait while we load your content...