A sustainable, efficient, and potentially cost-effective approach to the antimalarial drug candidate MMV688533†
Abstract
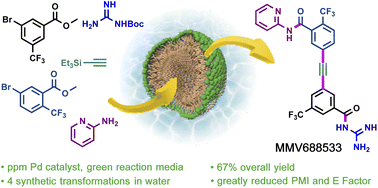
A 6-step synthesis of the antimalarial drug candidate MMV688533 is reported. Key transformations carried out under aqueous micellar conditions include two Sonogashira couplings and amide bond formation. Compared with the first-generation manufacturing process reported by Sanofi, the current route features ppm levels of palladium loading, less material input, less organic solvent, and no traditional amide coupling reagents. The overall yield is improved ten-fold, from 6.4% to 67%.


 Please wait while we load your content...
Please wait while we load your content...