Nitrogen-interrupted halo-Prins/halo-Nazarov fragment coupling cascade for the synthesis of indolines†
Abstract
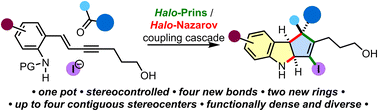
The nitrogen-interrupted Nazarov cyclization can be a powerful method for the stereocontrolled synthesis of sp3-rich N-heterocycles. However, due to the incompatibility between the basicity of nitrogen and the acidic reaction conditions, examples of this type of Nazarov cyclization are scarce. Herein, we report a one-pot nitrogen-interrupted halo-Prins/halo-Nazarov coupling cascade that joins two simple building blocks, an enyne and a carbonyl partner, to furnish functionalized cyclopenta[b]indolines with up to four contiguous stereocenters. For the first time, we provide a general method for the alkynyl halo-Prins reaction of ketones, thus enabling the formation of quaternary stereocenters. Additionally, we describe the outcomes of secondary alcohol enyne couplings, which exhibit helical chirality transfer. Furthermore, we investigate the impact of aniline enyne substituents on the reaction and evaluate the tolerance of different functional groups. Finally, we discuss the reaction mechanism and demonstrate various transformations of the prepared indoline scaffolds, highlighting their applicability in drug discovery campaigns.


 Please wait while we load your content...
Please wait while we load your content...