High-affinity single and double helical pseudofoldaxanes with cationic guests†
Abstract
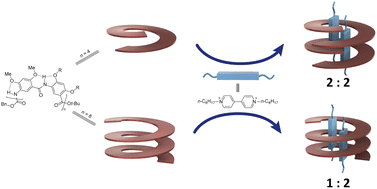
Two aromatic oligoamides, the 8-residue H8 and 16-residue H16, that adopt stable, cavity-containing helical conformations were examined for their complexation of a rodlike dicationic guest, octyl viologen (OV2+) and para-bis(trimethylammonium)benzene (TB2+). Studies based on 1D and 2D 1H NMR, isothermal titration calorimetry (ITC), and X-ray crystallography demonstrated that H8 and H16 wraps around two OV2+ ions as a double helix and a single helix, respectively, resulting in 2 : 2 and 1 : 2 complexes. Compared to H8, the longer H16 binds the OV2+ ions with much higher binding affinity and with extraordinary negative cooperativity. In contrast to its 1 : 2 binding with OV2+, the binding of helix H16 with the bulkier guest TB2+ shows a 1 : 1 ratio. Host H16 also selectively binds OV2+ in the presence of TB2+. This novel host–guest system features pairwise placement of the otherwise strongly repulsive OV2+ ions in the same cavity, strong negative cooperativity, and mutual adaptability of hosts and guests. The resultant complexes are highly stable [2]-, [3]-, and [4]pseudo-foldaxanes with few known precedents.
- This article is part of the themed collection: Celebrating the 70th Birthday and Achievements of David Lynn


 Please wait while we load your content...
Please wait while we load your content...