Unsymmetric N-heterocyclic carbene ligand enabled nickel-catalysed arylation of bulky primary and secondary amines†
Abstract
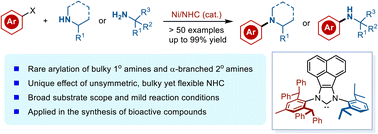
The arylation of sterically hindered amines represents one of the long-standing challenges in synthetic chemistry. Herein, we report a highly efficient Ni-catalysed arylation of sterically hindered primary and secondary amines with aryl chlorides or phenol derivatives enabled by an unsymmetric N-heterocyclic carbene (NHC) ligand. The protocol provides general, efficient, and scalable access to various sterically demanding anilines in excellent yields under mild conditions. A wide range of functional groups and heterocycles are compatible (>50 examples), including those present in biologically relevant molecules. Computational studies suggest that the unsymmetric bulky and flexible NHC ligand was critical to balance the oxidative addition and reductive elimination elementary steps, thus promoting this challenging transformation.


 Please wait while we load your content...
Please wait while we load your content...