Site-selective radical reactions of kinetically stable open-shell singlet diradicaloid difluorenoheteroles with tributyltin hydride and azo-based radical initiators†
Abstract
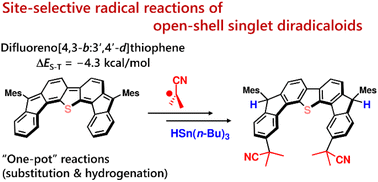
We have demonstrated site-selective radical reactions of the kinetically stable open-shell singlet diradicaloids difluoreno[3,4-b:4′,3′-d]thiophene (DFTh) and difluoreno[3,4-b:4′,3′-d]furan (DFFu) with tributyltin hydride (HSn(n-Bu)3) and azo-based radical initiators. Treatment of these diradicaloids with HSn(n-Bu)3 induces hydrogenation at the ipso-carbon in the five-membered rings, while treatment with 2,2′-azobis(isobutyronitrile) (AIBN) induces substitution at the carbon atoms in the peripheral six-membered rings. We have also developed one-pot substitution/hydrogenation reactions of DFTh/DFFu with various azo-based radical initiators and HSn(n-Bu)3. The resulting products can be converted into substituted DFTh/DFFu derivatives via dehydrogenation. Theoretical calculations unveiled a detailed mechanism of the radical reactions of DFTh/DFFu with HSn(n-Bu)3 and with AIBN, and that the site-selectivity of these radical reactions is controlled by the balance of the spin density and the steric hindrance in DFTh/DFFu.


 Please wait while we load your content...
Please wait while we load your content...