Structural snapshots of an Al–Cu bond-mediated transformation of terminal acetylenes†
Abstract
The copper(I) alumanyl derivative, [{SiNDipp}Al–Cu(NHCiPr)] (SiNDipp = {CH2SiMe2NDipp}2; Dipp = 2,6-di-isopropylphenyl; NHCiPr = N,N′-di-isopropyl-4,5-dimethyl-2-ylidene), reacts in a stepwise fashion with up to three equivalents of various terminal alkynes. This reactivity results in the sequential formation of cuprous (hydrido)(alkynyl)aluminate, (alkenyl)(alkynyl)aluminate and bis(alkynyl)aluminate derivatives, examples of which have been fully characterised. The process of alkene liberation resulting from the latter reaction step constitutes a unique case of alkyne transfer semi-hydrogenation in which the C–H acidic alkyne itself acts as a source of proton, with the Cu–Al bond providing the requisite electrons to effect reduction. This reaction sequence is validated by DFT calculations, which rationalise the variable stability of the initially formed heterobimetallic hydrides.
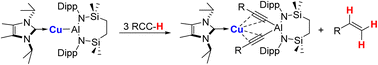


 Please wait while we load your content...
Please wait while we load your content...